Latest News
-
STAMI-SMI Professor Curtis's Lab Grows Natural Polymer Brushes from Surfaces Enzymatically
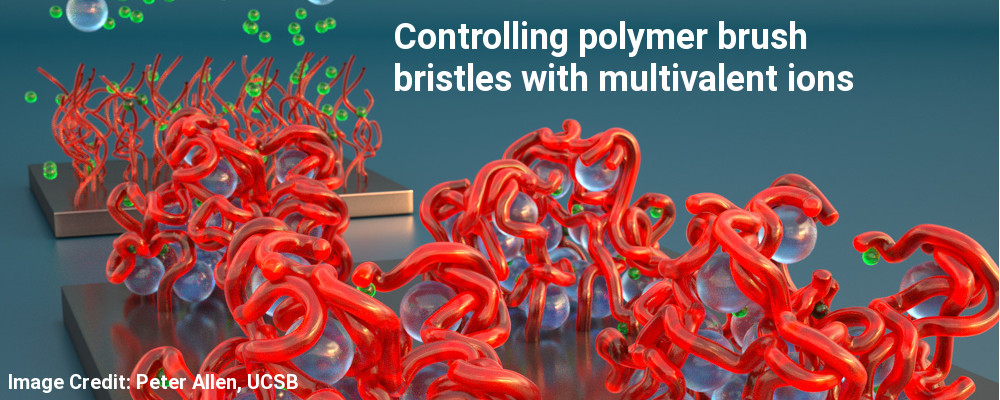
New work reported from the labs of SMI Professor Jennifer Curtis (School of Physics) introduces a versatile platform to grow ultra-thick, dense hyaluronan (HA) polymer brushes from surfaces using immobilized fragments of enzyme-rich bacterial membranes. This new method provides a path to improved biocompatibility and dynamic control of biointerfaces that potentially can be grown in vivo or regenerated after wear. The research was published in Nature Communications under open access (Nat. Commun. 2019, 10, 5527).
-
Solar Cells from STAMI Members Reach the International Space Station for Testing
Polymer and Perovskite solar cells fabricated in the labs of COPE Prof. Bernard Kippelen (Electrical and Computer Engineering) and COPE, GTPN Prof. Zhiqun Lin (Materials Science and Engineering) have been taken to the International Space Station (ISS) for testing in the harsh space environment as part of the MISSE-12 mission.
-
STAMI-COPE Co-Director Carlos Silva Elected to the Class of 2019 American Physical Society Fellows
STAMI-COPE Co-Director Carlos Silva, Professor in the School of Physics and the School of Chemistry and Biochemistry, has been elected to the 2019 Class of American Physical Society Fellows "[f]or the groundbreaking development of ultrafast laser techniques for probing the transient photophysics of electro-optical and excitonic materials leading to novel and unique insights into charge-separation and carrier generation in organic photovoltaic systems." Congratulations, Professor Silva!
-
STAMI-COPE Professor Shannon Yee Developing Thermoelectric Polymers for Personal Climate Control
STAMI-COPE Professor Shannon Yee and his team of Georgia Tech researchers are developing polymer-based thermoelectric (TE) materials for wearable devices to help people feel warmer or cooler on demand. The polymer TE materials could either harvest body heat to generate electricity or be used to produce a cooling sensation by hooking up a flexible battery to the circuitry.
-
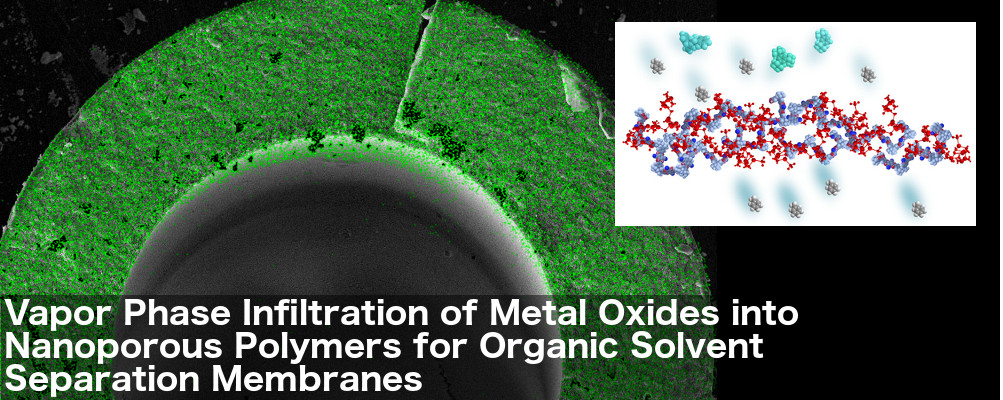
Metal Oxide-infused Membranes Could Offer Low-Energy Alternative For Chemical Separations
CRĀSI member Prof. Mark Losego and GTPN member Prof. Ryan Lively are working on membranes that could separate chemicals without using energy-intensive distillation processes in a wide variety of products including gasoline, plastics, and food. The latest work, published in Chemistry of Materials and sponsored by the Department of Defense and the National Science Foundation, outlined a process for taking a polymer-based membrane and infusing it with a metal oxide network. The resulting membrane is far more effective at standing up to harsh chemicals without degrading.